
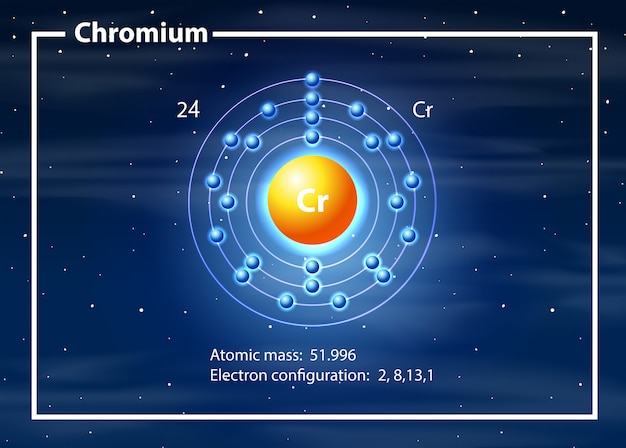
Chromium metal is valued for its high corrosion resistance and hardness. It is a steely-grey, lustrous, hard, and brittle transition metal. Chromium is a chemical element of the periodic table with chemical symbol Cr and atomic number 24 with an atomic weight of 51. In the d block, specifically the groups containing Chromium and Copper. Chromium is a chemical element with the symbol Cr and atomic number 24. Lide, (Ed.) in Chemical Rubber Company handbook of chemistry and physics, CRC Press, Boca Raton, Florida, USA, 81st edition, 2000. As we learned earlier, each neutral atom has a number of electrons equal to its.
Chromium atomic number full#
In these cases, a completely full or half full d sub-level is more stable than. Ley, Eds., Photoemission in Solids I: General Principles (Springer-Verlag, Berlin) with additional corrections, 1978. There are two main exceptions to electron configuration: chromium and copper. Burr, "Reevaluation of X-Ray Atomic Energy Levels," Rev. They are tabulated elsewhere on the WWW (reference 4) and in paper form (reference 5). The data are adapted from references 1-3. I am grateful to Gwyn Williams (Jefferson Laboratory, Virginia, USA) who provided the electron binding energy data. The binding energies are quoted relative to the vacuum level for rare gases and H 2, N 2, O 2, F 2, and Cl 2 molecules relative to the Fermi level for metals and relative to the top of the valence band for semiconductors. All values of electron binding energies are given in eV. 1967, 47, 1300.Įlectron binding energies Electron binding energies for chromium. The correct distribution of 3d electrons in the chromium present in the complex is.

These effective nuclear charges, Z eff, are adapted from the following references: Cr(H2O)6Cl3 (atomic number of Cr 24) has a magnetic moment of 3.83 B.M. In oxygen chromium is unstable and immediately it gives a thin layer of oxide and also protects the metal.Effective nuclear charges for chromium 1s The oxidation number is synonymous with the. When we heat chromium it turns into green chromic oxide. The oxidation state of an atom is the charge of this atom after ionic approximation of its heteronuclear bonds. The number of electrons in an atom is equal to the number of protons as atoms are electrically.
Chromium atomic number free#
It does not lose lustre when it is in free air. Therefore there are 24 protons in the nucleus of a chromium atom. Chromium is silver gray in color and it is highly polished. By the way in other words chromium is a brittle, lustrous and hard metal. The true statement(s) about chromium (atomic number 24 ) is/are. The atomic radius of a chemical element is a measure of the distance out to which the electron cloud extends from the nucleus. It must be noted, atoms lack a well-defined outer boundary. Advantages of Chromiumĭue to this ability of chromium, these are used in decoration and coating too. The atomic radius of Chromium atom is 139pm (covalent radius). The melting point is 2180 K while the boiling point is 2944 K. 5 Chromium metal is valued for its high corrosion resistance and hardness. It is a steely-grey, lustrous, hard, and brittle transition metal. Nicolas-Louis Vauquelin Related Topics: chemical element transition metal chromium processing See all related content chromium (Cr), chemical element of Group 6 (VIb) of the periodic table, a hard steel-gray metal that takes a high polish and is used in alloys to increase strength and corrosion resistance. The regular state of chromium is solid in real. Chromium is a chemical element with the symbol Cr and atomic number 24.
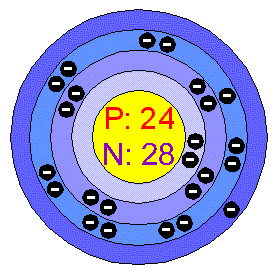
This surface is spinel in structure, it is very thick and also prohibits the diffusion of oxygen. Chromium is a chemical element with atomic number 24 and represented by the symbol Cr in the Periodic Table. Answer: From the periodic table, the atomic number of chromium is 24. When chromium is left in air it makes surface of steel non-reactive by altering the surface of metal through oxidation. Calculate the relative atomic mass of chromium. Learn about other important chemical elements such as Manganese, Silicon, Carbon which are used together with chromium for alloy making. When it went above 38 degree centigrade, it becomes paramagnetic i.e. Physical Properties of Chromium : Due to all the magnetic properties chromium is known because it is an elemental solid but in spite of this it shows anti-ferromagnetic ordering even in room temperatures.


 0 kommentar(er)
0 kommentar(er)
